- . Molar mass of NaCl = atomic mass of Na (22.99 amu) + the atomic mass of Cl (35.45 amu) 22.99 + 35.45 = 58.44 amu. One mol of NaCl (6.02 x10 23 formulas) has a mass of 58.44 g. The relation between molecular (formula) mass and molar mass.
- Relative formula mass (M r) is found by adding together the relative atomic masses (A r) of all the atoms present in a compound. Sodium Chloride is NaCl. The relative atomic masses can be found in the periodic table. A r of Cl: 35.5. M r of NaCl: 23 + 35.5 = 58.5.
Formula Weight For Nacl
. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. Molecular mass H 2 O = (2 x atomic mass of H) + atomic mass of O = 2(1.008 amu) + 16.00 amu = 18.02 amu. So, one mole of water (6.022 x 10 23 molecules) has a mass of 18.02 g. Molar mass of NaCl = atomic mass of Na (22.99 amu) +. Examples of molar mass computations: NaCl, Ca(OH)2, K4Fe(CN)6, CuSO4.5H2O, water, nitric acid, potassium permanganate, ethanol, fructose. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles. Calculate the no of moles in 50g of nacl. Take the atomic mass of CL as 35.5u - 6722171.
Molar Mass, Molecular Weight and Elemental Composition Calculator
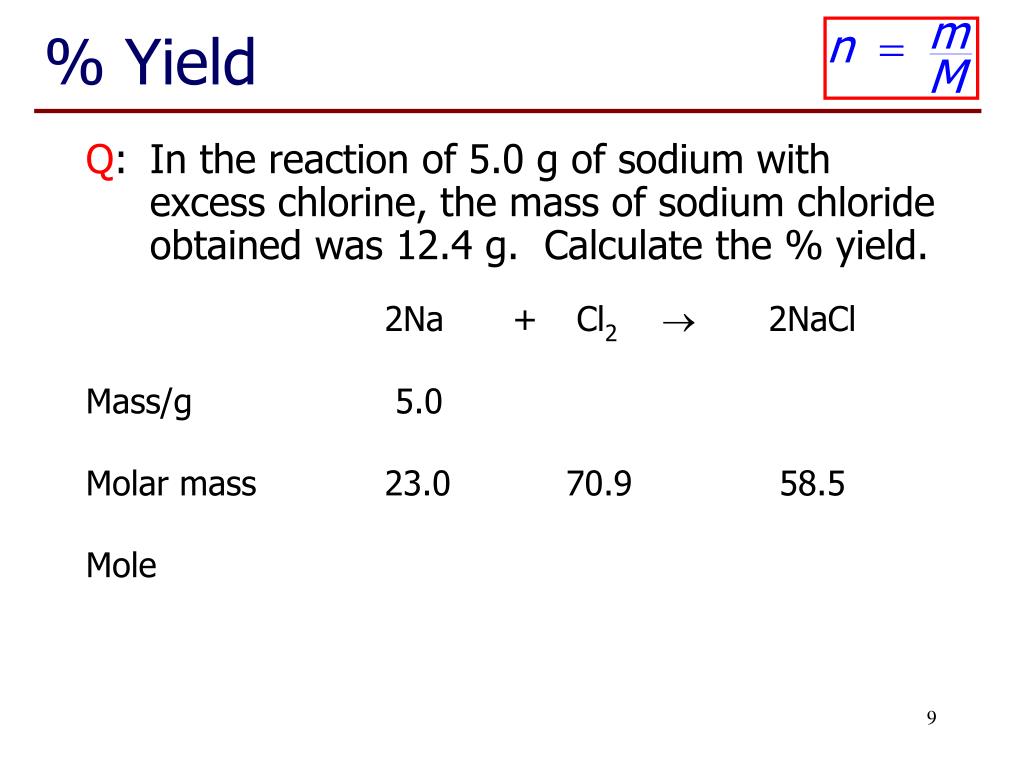
Finding Mass Of Solute
Molar mass of NaClO2 is 90.4416 g/mol Compound name is sodium chlorite Convert between NaClO2 weight and moles
Elemental composition of NaClO2
Sample reactions for NaClO2
Formula in Hill system is ClNaO2 | ||||||||||||||||||||||||||||||||||||
Computing molar mass (molar weight)To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use:
Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. Computing molecular weight (molecular mass)To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.Examples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. Definitions of molecular mass, molecular weight, molar mass and molar weight
Give us feedback about your experience with Molecular Weight Calculator. Related: Molecular weights of amino acids | ||||||||||||||||||||||||||||||||||||
| molecular weights calculated today | ||||||||||||||||||||||||||||||||||||
Atomic Mass Of Nacl2
| Back to Online Chemical Tools Menu |

© 2021 webqc.org All rights reserved

| Periodic table |
| Unit converters |
| Chemistry tools |
| Chemical Forum |
| Chemistry FAQ |
| Constants |
| Symmetry |
| Chemistry links |
| Link to us |
| Contact us |
How to cite? |
WebQC.Org online education free homework help chemistry problems questions and answers |
